Innovative Endovascular Technologies, LLC was incorporated as a Skolkovo Foundation liability corporation. The R&D is outsourced to Northeast Biomedical, a product development firm in Tyngsboro, MA, US, and Angiolain, Novosibirsk, Russia.
Dr. Viller has >20 years of experience within interventional cardiology. Alongside his practice, he teaches in the Department of Thoracic and Cardiovascular at the NI Pirogov and holds multiple patents within his field.
The intellectual property covers the use of a double balloon stent delivery system on a bifurcated coronary lesion.
Invention makes it possible to implant truncated stent in precise way due to clear visualisation of X-ray contrast marks of "signal balloon", located in one plane with maximal and minimal stent faces.
Innovative Endovascular Technologies, LLC was incorporated in 2009 dedicated to helping interventional cardiologists and interventional radiologists perform successful coronary stenting procedures.
Owned and operated by Alexander G. Viller, MD, PhD the head of interventional cardiology at Priogov National Surgery Center, Moscow Russia with +20 yrs. of interventional cardiology experience.




The VILLER stent system consists of a double-balloon delivery system used to implant an eccentric bare metal stent (BMS) in lesions located in a bifurcated region or unstable plaques with eccentric morphology.
Historically, bifurcations have been have been associated with lower procedural success rates and worse clinical outcomes than when used to treat non-bifurcation lesions due to the increased technical complexity and lack of data on treatment methods. While most stents are designed for non-bifurcated lesions, this stent has specialized edges to conform more closely to the vessel’s structure. Unstable plaques also present a major challenge in interventional cardiology. While imaging of these plaques has been a major focus of research and has advanced greatly over the past decade, treatment of unstable plaques remains a major clinical challenge.
The above problems are solved by creation of an advanced design of the delivery system consisting of a multilumen polymeric catheter, of which the distal end is equipped with two polymeric balloons arranged in-line, the distal one for expansion of an eccentric stent mounted on it, and the proximal one, having smaller diameter, supplied with radiopaque markers, for exect 3D positioning of the stent in an arterial lumen under fluoroscopy control.
The Viller stent system consists of a double-balloon delivery system used to implant an eccentric stent in bifurcated lesions.
The advantages over similar existing interventional cardiology treatments offered by the VILLER stent include:
The initial product under development at Viller Scientific, is the VILLER stent system. This product consists of a double-balloon delivery system used to implant an eccentric bare metal stent (BMS) in lesions located in a bifurcation region. In future generations a DES can be substituted for a BMS.
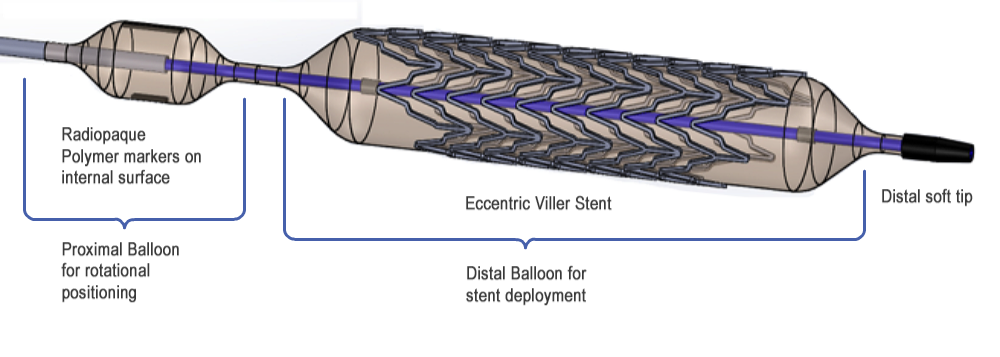
Figure 1. Distal end of VILLER stent delivery system showing novel design features (stent and balloon are shown in expanded configuration).
The design includes a “signal” balloon slightly proximal to the stent delivery balloon as shown in Figure 3, of a slightly smaller diameter. The stent delivery system has a single lumen used for the inflation of both balloons. During the stent delivery procedure, the signal balloon is inflated to a low pressure after the stent deployment balloon has been placed in the vicinity of the lesion. This low pressure is not enough to deploy the stent, but will inflate the signal balloon so that the radiopaque markers at its outer surface are clearly visible through fluoroscopy and can be used to rotationally position the eccentric stent. Once properly positioned, the stent will then be deployed by increasing the pressure within both balloons. Due to the non-compliant nature of the signal balloon, its diameter will remain smaller than the stent deployment balloon even at high pressures to prevent damage to the arterial wall in the adjacent region.
This product utilizes many standard components from rapid exchange stent delivery systems; however it differentiates itself through its novel solution for bifurcation stenting, Figure 3. The novel design features an eccentric Cobalt Chromium alloy stent in the shape shown in Figure 2 for use at bifurcations in the orientation depicted in Figure 3.
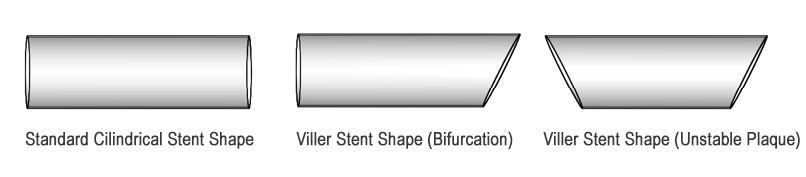
Figure 2. Shape of a standard coronary stent vs. the VILLER coronary stents for bifurcation regions and unstable plaques.
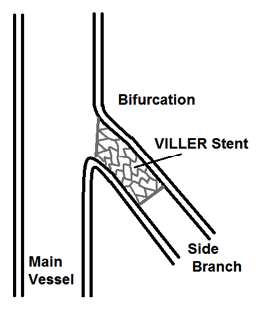
Figure 3. Final placement of VILLER within side branch of vessel bifurcation region
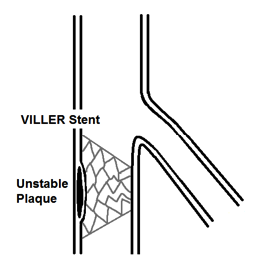
Figure 4. Final placement of VILLER stent for unstable plaque.
Patents have been issued on this design in the United States, EU and Asia. The intellectual property covers the use of a double balloon stent delivery system on a bifurcated coronary lesion. The invention relates to medical appliances, namely, to devices used in endovascular surgery and interventional cardiology for restoration of narrowed bifurcation sites of a lumen of blood vessels, and also for treatment of an artery in the presence of an unstable atherosclerotic plaque for prevention of its rupture and an acute atherothrombosis, which is a basic etiological factor of an acute myocardial infarction. The details of the issued patents and applications will be disclosed to potential investors during the initial stages of due diligence.